
NeuroPro® Rigid Fixation System
Why Choose the NeuroPro System?
With each component carefully engineered for flawless performance and efficient use in the OR, the NeuroPro System makes flap closure a pleasure instead of a chore. Designed specifically for neurosurgical applications, the NeuroPro System addresses all your rigid fixation requirements, including cranioplasty and skull base applications, in a single compact modular tray. This coupled with the documented performance of titanium plate and screw fixation for long-term flap stability1,2,3, enables you to provide the best fixation option for your patients.
 The unique three level autoclave tray contains everything needed for all neurosurgical rigid fixation procedures. A complete selection of titanium plates, panels and screws is carefully organized and labeled for maximum efficiency and ease of use in the OR. All necessary drill bits and instruments are also organized in the tray.
The unique three level autoclave tray contains everything needed for all neurosurgical rigid fixation procedures. A complete selection of titanium plates, panels and screws is carefully organized and labeled for maximum efficiency and ease of use in the OR. All necessary drill bits and instruments are also organized in the tray.
 An asymmetric burr hole cover design allows flexibility in positioning of the plate such that screws can be placed away from the edge of craniotomy cuts. At least two screws can always be placed in the flap — even with closely spaced cuts.
An asymmetric burr hole cover design allows flexibility in positioning of the plate such that screws can be placed away from the edge of craniotomy cuts. At least two screws can always be placed in the flap — even with closely spaced cuts.
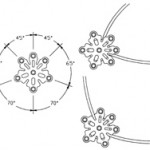
Patented burr hole cover design with central port provides for passage of ICP monitors or other catheters. With the catheter routed through the burr hole cover it is less likely to contact sharp bone edges and may be easier to remove. The port edges are rounded to reduce the potential for damage to the catheter.
 The Gap Panel in the set is used to cover gaps that arise along a repositioned or widened bone flap thereby protecting underlying structures and preventing cosmetic deformity.
The Gap Panel in the set is used to cover gaps that arise along a repositioned or widened bone flap thereby protecting underlying structures and preventing cosmetic deformity.
 The Quick Tap® Power Driver is precisely engineered to provide optimum speed and torque for efficiently driving Kinamed’s Quick Tap self-drilling screws. Well thought-out ergonomics and a sleek design make the driver a pleasure to use. In addition, all issues of reliability and sterile processing are completely eliminated by virtue of the single-use, sterile-packaged design. The low cost of the Quick Tap Driver may be less than the hospital cost of reprocessing, cleaning and sterilizing a re-useable battery driver.
The Quick Tap® Power Driver is precisely engineered to provide optimum speed and torque for efficiently driving Kinamed’s Quick Tap self-drilling screws. Well thought-out ergonomics and a sleek design make the driver a pleasure to use. In addition, all issues of reliability and sterile processing are completely eliminated by virtue of the single-use, sterile-packaged design. The low cost of the Quick Tap Driver may be less than the hospital cost of reprocessing, cleaning and sterilizing a re-useable battery driver.
The following issues that occur with re-useable battery drivers are completely eliminated:
- Availability of a sterile processed unit at the time of surgery.
- Failure at the time of surgery due to faults caused by multiple autoclaving.
- Availability of re-charged or replacement batteries
- Concerns about proper cleaning and sterile processing.
 Both Quick Tap self-drilling screws and standard screws are available providing surgeons with a choice of either system. Quick Tap screws utilize a sophisticated design to ensure reliability and deliver upon the potential of the self-drilling screw concept. Made from high strength titanium alloy, they are uniquely engineered to minimize insertion force and prevent stripping during insertion.
Both Quick Tap self-drilling screws and standard screws are available providing surgeons with a choice of either system. Quick Tap screws utilize a sophisticated design to ensure reliability and deliver upon the potential of the self-drilling screw concept. Made from high strength titanium alloy, they are uniquely engineered to minimize insertion force and prevent stripping during insertion.
 All plates, including the burr hole covers, have beveled edges to reduce the possibility of soft tissue irritation or plate palpability under the scalp.
All plates, including the burr hole covers, have beveled edges to reduce the possibility of soft tissue irritation or plate palpability under the scalp.
 The unique NeuroPro taper fit screwdriver is engineered to easily pick up screws and reliably drive them without becoming disengaged prior to complete seating. The positive grip on the screw virtually eliminates problems of dropping, premature disengagement and stripping of the screw. (Patent pending).
The unique NeuroPro taper fit screwdriver is engineered to easily pick up screws and reliably drive them without becoming disengaged prior to complete seating. The positive grip on the screw virtually eliminates problems of dropping, premature disengagement and stripping of the screw. (Patent pending).
 The rotary screw organizer lid opens to the specific screw size required and prevents screw spillage. Screws are easily picked up with the NeuroPro taper fit driver and are made from high strength, strip resistant titanium alloy.
The rotary screw organizer lid opens to the specific screw size required and prevents screw spillage. Screws are easily picked up with the NeuroPro taper fit driver and are made from high strength, strip resistant titanium alloy.
 A complete selection of drill bits, including Anspach* and Midas Rex* compatibles, are available in the set. The cost and inconvenience of setting up a second drill in the OR to drill screw pilot holes when using the standard screws can be eliminated.
A complete selection of drill bits, including Anspach* and Midas Rex* compatibles, are available in the set. The cost and inconvenience of setting up a second drill in the OR to drill screw pilot holes when using the standard screws can be eliminated.
 The panel implant has a unique angled connecting bar geometry (patented) that allows for 3-D contouring to match the spherical radii on the cranium in cranioplasty reconstructions. Special instruments in the tray facilitate easy and accurate shaping of the panel.
The panel implant has a unique angled connecting bar geometry (patented) that allows for 3-D contouring to match the spherical radii on the cranium in cranioplasty reconstructions. Special instruments in the tray facilitate easy and accurate shaping of the panel.
*Midas Rex is a registered trademark of Midas Rex Pneumatic Tools, Inc.; *Anspach is a registered trademark of the Anspach Effort, Inc. NeuroPro® and Quick Tap® are trademarks of Kinamed,® Inc.
TMS Plate™
Temporalis Muscle Suspension Plate
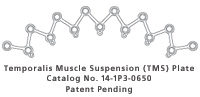
Craniotomies in the temporal and pterional region often require detachment of the temporalis muscle from the bone. If the muscle is not re-approximated and secured in its correct anatomical position, post-operative contracture of the muscle can occur resulting in masticatory dysfunction and cosmetic deformity.
The Temporalis Muscle Suspension (TMS) Plate has been designed to allow rapid, efficient, and secure fixation of the superior border of the temporalis muscle to the superior temporal line. Instead of taking the time to drill tangential holes in this thin area of the skull, and to avoid placing screws directly through the muscle fibers, this technique provides multiple points for suture attachment along the suspension plate. This multiplicity of suture attachment points helps to distribute loads and provides suspension across a wide area of the muscle. At the same time, the plate may be used to bridge the craniotomy gap, thus providing the required rigid fixation along that side of the bone flap.
Reference list
- Smith, S.C.; Pelofsky, S: Adaptation of Rigid Fixation to Cranial Flap Replacement. Neurosurgery 29:417-418, 1991.
- Chandler, C.L., et al: Imaging after titanium cranioplasty. Br J Neurosurgery 8:409-414, 1994.
- Malis, L.I.: Titanium Mesh and Acrylic Cranioplasty. Neurosurgery 25:351-355, 1989.
Patents
U.S. Patent Nos. 5,980,540, 5,961,519, 8,662,299, D534,651. Japan Patent No. 5,192,371. Additional US and World Patents Pending
